This workbook consists of introductory reading and five distinct modules that encourage you to reflect on your CVD risk assessment and management practices, risk communication, cultural safety, and your engagement with young adult Māori and Pacific patients.
Rosuvastatin newly funded for management of cardiovascular disease and familial hypercholesterolaemia
Rosuvastatin is fully funded in New Zealand from 1 December 2021, subject to Special Authority criteria. This means that those at increased risk of cardiovascular complications due to high cholesterol now have access to a fourth funded HMG-CoA reductase inhibitor, or statin.1 Any relevant practitioner, including nurse practitioners and pharmacist prescribers, can apply for a Special Authority.
Contents
- Introduction and Special Authority criteria
- Is my patient eligible for funded rosuvastatin?
- Place in therapy
- Beginning treatment with rosuvastatin
- Potential side effects of statins
- Drug interactions – effects of co-administered medications on rosuvastatin
- Drug interactions – effects of rosuvastatin on co-administered medications
- Patient information
Click here to view/print an abridged PDF version of this article.
Introduction and Special Authority criteria
In an agreement with Viatris Ltd (previously Mylan New Zealand Ltd), the brand Rosuvastatin Viatris will be funded in 5mg, 10mg, 20mg and 40mg once-daily tablet doses. The full funding criteria for patient eligibility have been detailed by Pharmac1 and are summarised below.
Criteria have been applied to allow funded access for patients with:
- raised cardiovascular disease (CVD) risk
- familial hypercholesterolaemia
- established CVD
- recurrent major cardiovascular events.
Patients with the above specified risks/condition(s), as defined in the full criteria, must also have reached a maximum tolerated dose of their atorvastatin and/or simvastatin treatment and have a low-density lipoprotein cholesterol (LDL-C) level still above target for that condition. Note that the targets specified in the criteria are in line with international guidelines and may differ from those used locally.
In addition to these clinical criteria, two further specific criteria apply:
- a pro-equity eligibility component for people of Māori or Pacific ethnicity with a higher risk of CVD, to access rosuvastatin as a first-line treatment option
- a waiver process for patients already self-funding rosuvastatin.
Māori and Pacific peoples are at a higher overall risk of CVD compared with most other ethnicities in New Zealand. Ministry of Health data show Māori to have total CVD mortality rates more than two-fold those of non-Māori and a 1.5 times likelihood compared with non-Māori of being hospitalised for CVD.2 Similarly, CVD is the principal cause of death for Pacific peoples, and cardiovascular mortality rates are consistently and significantly higher than for the general population.3
For Māori and Pacific populations, CVD risk assessment is recommended to begin in men at age 30, and in women at age 40 (15 years earlier than other population groups).4 As such, rosuvastatin is funded for first-line use in eligible Māori and Pacific peoples with raised CVD risk.1
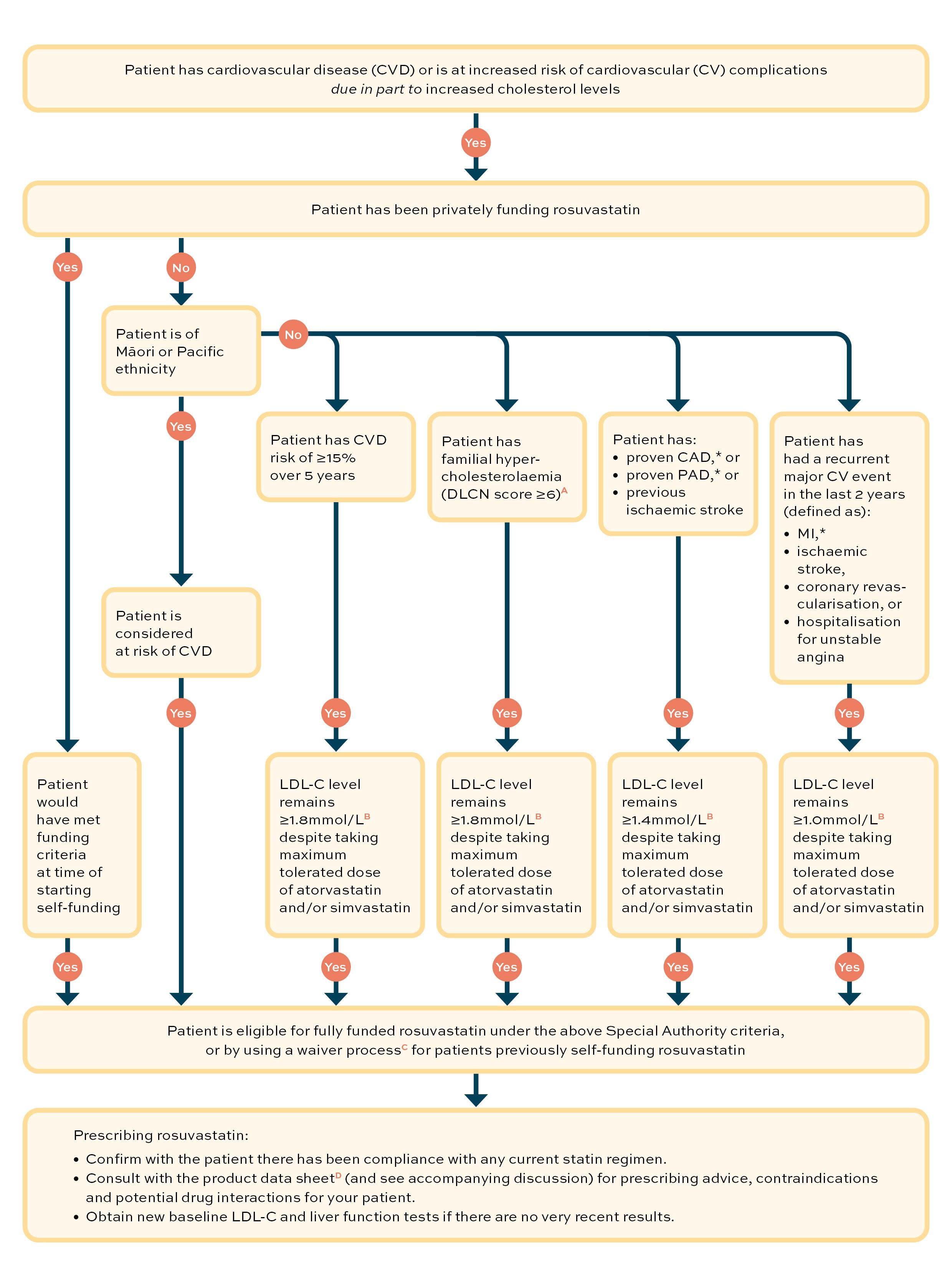
Is my patient eligible for funded rosuvastatin?
Rosuvastatin is a funded option for lipid-lowering therapy in New Zealand for patients with cardiovascular disease or at risk of cardiovascular disease from 1 December 2021, subject to Special Authority criteria.
This flow diagram can be used to guide your decision as to whether or not your patient is eligible for funded treatment with rosuvastatin.
Key:
(A) Dutch Lipid Clinical Network Score (DLCNS), calculator. www.athero.org.au/fh/calculator
(B) The low-density lipoprotein (LDL-C) level targets used in these Special Authority criteria are in line with international guidelines
(C) Pharmac. Special Authority waiver. https://pharmac.govt.nz/medicine-funding-and-supply/make-an-application/special-authority-waiver
(D) Medsafe. New Zealand data sheet. Rosuvastatin Viatris. Revised: 23 August 2021. Available online at www.medsafe.govt.nz/profs/Datasheet/r/rosuvastatinviatristab.pdf
* CAD = coronary artery disease; PAD = peripheral artery disease; MI = myocardial infarction
Place in therapy
Statins are the preferred choice of lipid-lowering drug as they consistently reduce morbidity and mortality across a wide range of population subgroups, regardless of blood cholesterol levels. For each 1mmol/L reduction in LDL-C, trials demonstrate that patients achieve an approximate 25 per cent relative risk reduction in CVD events over five years. Further, no trial has demonstrated an LDL-C level below which further lowering is not associated with further reduction in CVD risk.4
Rosuvastatin is the most potent of the four funded statins and achieves an equivalent therapeutic effect at a lower dose than the other available statins.4
Rosuvastatin reduces the LDL-C level across its dose range and, milligram-for-milligram, it is three to four times as potent as atorvastatin.
All statins act by limiting the rate of enzymatic cholesterol synthesis in the liver. These agents also upregulate LDL-C receptor expression, increasing LDL clearance from the circulation. Statins reduce blood levels of total cholesterol, LDL-C and, to a lesser extent, triglycerides. Statins may also reduce inflammation and have atherosclerotic plaque stabilisation effects.5
Any available statin may be initiated to reduce LDL-C levels and, if the target is met and the statin is tolerated, there is no need to change treatment. Rosuvastatin can be considered when another statin has been ineffective in achieving the target LDL-C level. Higher doses of rosuvastatin (20–40mg) achieve reductions in LDL-C not possible with most of the recommended doses of other statins.6 While the toxicity profile of rosuvastatin is similar to that of other statins,6 some prescribing precautions at higher doses need to be noted (see Potential side effects).
In addition to the increased potency of rosuvastatin in lowering LDL-C levels, it has also been shown to elevate high-density lipoprotein cholesterol (HDL-C) levels by 8–10 per cent over 6 weeks, compared with approximately 2–6 per cent for atorvastatin, 5–7 per cent for simvastatin and 3–6 per cent for pravastatin;7 and these effects are across its recommended dose range, unlike atorvastatin.8 Reductions in triglyceride levels with rosuvastatin are similar to those with atorvastatin across the dose ranges and greater than those produced with simvastatin and pravastatin.7,8
With regard to the important lipid ratios, rosuvastatin 10mg has been shown to improve total cholesterol/HDL-C, LDL-C/HDL-C and non-HDL-C/HDL-C ratios compared with atorvastatin 10mg, simvastatin 10–40mg and pravastatin 10–40mg.8,9
From 1 December 2021, rosuvastatin is funded on Special Authority for patients at increased risk of cardiovascular complications due to high cholesterol levels (see flow diagram). Part of the criteria for funding is that current treatment targets have not been met using a maximum tolerated dose of another statin. Two points are worthy of note:
- the targets for LDL-C treatment are aligned with international guidelines and may not be the same as New Zealand LDL-C targets for any group of patients
- it is the prescriber’s clinical judgement whether the patient tolerates their statin dose.
Within the Special Authority criteria, the cut-offs for funded rosuvastatin treatment for patient groups on a maximum tolerated dose of atorvastatin and/or simvastatin are:
- with a calculated risk of CVD of ≥15% over five years, LDL-C level remains ≥1.8mmol/L
- with familial hypercholesterolaemia (Dutch Lipid Clinic Network score ≥6), LDL-C level remains ≥1.8mmol/L
- with coronary artery disease, proven peripheral artery disease or ischaemic stroke, LDL-C level remains ≥1.4mmol/L
- with a recurrent major cardiovascular event (myocardial infarction, ischaemic stroke, coronary revascularisation, hospitalisation for unstable angina) in the last two years, LDL-C level remains ≥1.0mmol/L.
(Note: there is funding eligibility for first-line treatment for people of Māori or Pacific ethnicity at risk of CVD, and a waiver process for those previously self-funding rosuvastatin who would have met the Special Authority criteria at the time of starting it.)
Beginning treatment with rosuvastatin
Statins are first-line treatment for the reduction of LDL-C in patients whose levels remain above target after dietary and lifestyle modification. If LDL-C targets are not achieved with the maximum tolerated dose of a statin, a change to a more potent statin can be initiated (once patient adherence with treatment has been confirmed) or the addition of a non-statin, lipid-lowering agent in appropriate circumstances.6
Switching statins should not incur any significant delay – the complete withdrawal of statins can lead to a rapid rise in cholesterol levels over as little as two weeks. When switching a patient from one statin to another, the changeover should be managed in much the same way as statin therapy is initiated.10
Rosuvastatin, like atorvastatin, can be taken at any time of day, with or without food,11 as both have a longer half-life than simvastatin and pravastatin, which need to be taken in the evening (as endogenous cholesterol synthesis peaks overnight during a period of fasting).12 There is minimal accumulation of rosuvastatin on repeated once-daily dosing.11,13
The Medsafe data sheet for Rosuvastatin Viatris states:11
- a dose of 20mg once daily reduces the risk of major cardiovascular events
- for hypercholesterolaemia, the usual dose range is 10–40mg once daily, individualised to goal of therapy and patient response; most patients are controlled at the start dose, but adjustments can be made at two to four-week intervals
- for primary hypercholesterolaemia (including heterozygous familial hypercholesterolaemia), mixed dyslipidaemia and isolated hypertriglyceridaemia, the usual start dose is 10mg once daily
- for patients with severe hypercholesterolaemia (including heterozygous familial hypercholesterolaemia), a start dose of 20mg once daily may be considered
- for patients with homozygous familial hypercholesterolaemia, a start dose of 20mg once daily is recommended
- a dose of 40mg once daily should only be considered in patients still at high cardiovascular risk after assessing their response to a dose of 20mg. A 40mg dose is used only in patients in whom regular follow-up is planned, and the 40mg dose must not be exceeded in any patient.11 (Use of doses above 20mg should ideally involve discussion with a specialist.)5,6
A response to treatment with rosuvastatin (reduced LDL-C level) is evident within one week of starting, and 90 per cent of maximum response is usually achieved by two weeks of treatment.11,13
It is reasonable to consider starting some patients with the rosuvastatin 5mg once-daily dose, which can be titrated if necessary to meet treatment goals.6 The 5mg starting dose is advised for some Asian populations (Filipino, Chinese, Japanese, Korean, Vietnamese or Asian-Indian origin) who tolerate rosuvastatin less well due to a two-fold higher systemic exposure when compared with Caucasian populations.11,13 Prescribers should be cautious when titrating rosuvastatin and should not use the 40mg dose in this group.6,11
The usual dose range applies in the elderly, and in patients with mild-to-moderate renal impairment and mild-to-moderate hepatic impairment. There are specific recommendations for severe renal and hepatic impairment and certain genetic polymorphisms.11 A small number of contraindications include active liver disease or persistent, unexplained elevations in transaminases; pregnancy and breastfeeding; with an additional list of contraindications (predisposing factors for myopathy/rhabdomyolysis) for the highest 40mg dose – see full data sheet.11
Liver function tests should be performed before initiating rosuvastatin and periodically thereafter. Raised transaminases should be monitored until resolved (dose reduction or withdrawal of rosuvastatin is recommended for persisting ALT or AST levels of more than three times the upper limit of normal).11 Excess alcohol consumption is a further precaution, as is a history of liver disease and uncorrected hypothyroidism.11
Potential side effects of statins
Statins are generally well tolerated. Large-scale randomised controlled trials report similar discontinuation rates to placebo.11 Serious side effects from long-term statin therapy are rare.4 For every 10,000 patients treated for five years, there are:
- five cases of myopathy
- 50 to 100 new cases of diabetes (the reduction in CVD events outweighs any related harm).4
Other common minor side effects include gastrointestinal disturbances, altered liver function (transaminases should be tested prior to starting and monitored periodically throughout treatment with rosuvastatin), sleep disturbance and headache.5,6,11 Often, raised transaminase levels resolve over time without statin dose adjustment.14 Importantly, there has been no link between statin-associated elevation of liver enzymes (up to three times ULN) and actual liver toxicity or disease.14
Skeletal muscle effects – as with other statins, effects on skeletal muscle (eg, myalgia, myopathy and, rarely, rhabdomyolysis) have been reported with rosuvastatin use.11 Myalgia is reported commonly in patients receiving statins; however, muscle toxicity truly attributable to statin use is rare – the likelihood increases with higher doses and in certain patients.15 Patients who develop any signs or symptoms suggestive of myopathy should have their creatine kinase levels measured and rosuvastatin stopped if markedly elevated (creatine kinase >10 times ULN), or if myopathy is diagnosed or suspected.11
While muscle toxicity has been reported with all statins, lipophilic statins (simvastatin, atorvastatin) penetrate muscle more easily than hydrophilic statins (pravastatin) and are associated with a higher incidence of adverse effects, particularly myopathy. Rosuvastatin is a “relatively hydrophilic” statin.14,16 While hydrophilic statins have a lower association with adverse effects, they generally require higher dosing to be efficacious, with the exception of rosuvastin.14,16 Higher doses, in turn, may be associated with adverse effects.
See the New Zealand Formulary5 and available data sheets11,13 for a full description of rosuvastatin possible side effects.
Drug interactions – effects of co-administered medications on rosuvastatin
In contrast to simvastatin and atorvastatin, rosuvastatin has no clinically significant cytochrome P450 interactions (as a substrate, inhibitor or inducer).11 This has been confirmed in studies with known cytochrome P450 3A4 inhibitors (ketoconazole, erythromycin, itraconazole).11,13 Rosuvastatin has minimal CYP2C9 metabolism but is largely excreted unchanged.14
Rosuvastatin is a substrate for certain transporter proteins including the hepatic uptake transporter OATP1B1 and efflux transporter BCRP. Concomitant administration of rosuvastatin with inhibitors of these transporters may result in increased rosuvastatin plasma concentrations and an increased risk of myopathy. Dose adjustments for rosuvastatin, or recommendations to avoid concurrent use, are outlined in the data sheet for a range of medications, including:11
- protease inhibitors (antivirals) – may increase rosuvastatin concentrations up to seven-fold
- cyclosporin – may increase rosuvastatin concentrations up to seven-fold
- gemfibrozil – may increase rosuvastatin concentrations up to two-fold
- clopidrogel – may increase rosuvastatin concentrations up to two-fold
- erythromycin – may decrease rosuvastatin concentrations by as much as 28 per cent.
Other interacting medicinal products include antacids and fusidic acid. Antacids can halve the rosuvastatin plasma concentration, but the effect is mitigated when the antacid is given two hours after rosuvastatin.11 The risk of myopathy, including rhabdomyolysis, may be increased by the concomitant administration of systemic fusidic acid with a statin. Co-administration may cause increased plasma concentrations of both agents – the mechanism for this interaction is as yet unknown. If treatment with fusidic acid is necessary, rosuvastatin treatment should be discontinued throughout the duration of the fusidic acid treatment.13
See the New Zealand Formulary, Stockley’s Alerts5 and available data sheets11,13 for a full description of rosuvastatin medication interactions.
Drug interactions – effects of rosuvastatin on co-administered medications
While warfarin pharmacokinetics are not significantly affected by rosuvastatin, co-administration of rosuvastatin may result in a rise in INR compared with warfarin alone (as is the case with other statins). In patients taking vitamin K antagonists, it is important to monitor for increased INR at the initiation or cessation of rosuvastatin therapy and following dose adjustment.5,6,11
Gemfibrozil, fenofibrate and other fibric acids, including nicotinic acid, may increase the risk of myopathy when given concomitantly with statins. No pharmacokinetic interaction between rosuvastatin and fenofibrate has been observed; however, a pharmacodynamic interaction may occur.11
See the New Zealand Formulary, Stockley’s Alerts5 and available data sheets11,13 for a full description of rosuvastatin medication interactions.
Patient information
New Zealand prescribers and patients may well have experience with rosuvastatin, as it has been available, privately funded, for at least 15 years. Statin adverse effect profiles are similar6 and switching to rosuvastatin is an option for more vigorous treatment to targets.
Patient information on statins and rosuvastatin is available at:
- Heart Foundation. Statins: www.heartfoundation.org.nz/your-heart/heart-treatments/medications/statins
- Medsafe. Consumer Medicine Information, rosuvastatin (Rosuvastatin Viatris): www.medsafe.govt.nz/consumers/CMI/r/rosuvastatinviatris.pdf
- Health Navigator. Rosuvastatin: www.healthnavigator.org.nz/medicines/r/rosuvastatin/
- NPS MedicineWise. Consumer Medicine Information, rosuvastatin (Apo-Rosuvastatin): www.nps.org.au/medicine-finder/apo-rosuvastatin#cmi
- NHS. Medicines A–Z. Rosuvastatin: www.nhs.uk/medicines/rosuvastatin/
Acknowledgements
Written by: Richard French (BSc), freelance medical writer and regular contributor to He Ako Hiringa resources
Reviewed by: Dr Fraser Hamilton (MBChB, FRNZCGP)
Animated videos by: Alice McRae, clinical writer He Ako Hiringa
Professional college endorsements
This activity has been endorsed by The Royal New Zealand College of General Practitioners (RNZCGP) and has been approved for up to 0.25 CME credits for continuing professional development purposes (1 credit per learning hour). To claim your CPD credits, log in to your Te Whanake dashboard and record these activities under the appropriate learning category.
This activity has been endorsed by the PSNZ as suitable for inclusion in a pharmacist’s CE records for CPD purposes.
References (for this article)
All references were accessed in September/October 2021.
1. Pharmac. Decision to fund rosuvastatin for people with high cholesterol. 17 August 2021. Available online at https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/2021-08-17-decision-rosuvastatin
2. Ministry of Health. Maori Health. Ngā mana hauora tūtohu: Health status indicators. Cardiovascular disease. Available online at www.health.govt.nz/our-work/populations/maori-health/tatau-kahukura-maori-health-statistics/nga-mana-hauora-tutohu-health-status-indicators/cardiovascular-disease
3. Ministry of Health. Pacific people's health. Available online at www.health.govt.nz/our-work/populations/pacific-health/pacific-peoples-health
4. Ministry of Health. 2018. Cardiovascular disease risk assessment and management for primary care. Wellington: Ministry of Health. Available online at www.health.govt.nz/system/files/documents/publications/cardiovascular-disease-risk-assessment-management-primary-care-feb18-v4_0.pdf
5. New Zealand Formulary. Rosuvastatin product monograph. Available online at https://nzf.org.nz/nzf_1616
6. NPS Medicinewise. RADAR: Rosuvastatin (Crestor) for dyslipidaemia rosuvastatin. 1 December 2006. Available online at www.nps.org.au/radar/articles/rosuvastatin-crestor-for-dyslipidaemia
7. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 2003;92(2):152–60. Available online at www.sciencedirect.com/science/article/abs/pii/S0002914903005307
8. Tuomilehto J. Rosuvastatin: the most efficient treatment option for patients with dyslipidemia. Future Lipidology 2007;2(2):127–41. DOI: 10.2217/17460875.2.2.127. Available online at www.tandfonline.com/doi/pdf/10.2217/17460875.2.2.127
9. Jones PH, Hunninghake DB, Ferdinand KC et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther 2004;26(9):1388–99. Available online at www.clinicaltherapeutics.com/article/S0149-2918(04)80285-6/pdf
10. Medsafe. Prescriber Update 16:36. Dose titration recommended when switching statins. July 1998. Available online at www.medsafe.govt.nz/profs/PUarticles/12.htm
11. Medsafe. New Zealand data sheet. Rosuvastatin Viatris. Revised: 23 August 2021. Available online at www.medsafe.govt.nz/profs/Datasheet/r/rosuvastatinviatristab.pdf
12. Awad K, Serban M, Penson P, et al. Effects of morning vs evening statin administration on lipid profile: A systematic review and meta-analysis. J Clin Lipidol 2017;11:972–85. Available online at www.lipidjournal.com/article/S1933-2874(17)30343-4/fulltext
13. NPS MedicineWise. APO-Rosuvastatin product information. Available online at www.nps.org.au/medicine-finder/apo-rosuvastatin#full-pi
14. Howe CL. POGOE: The Portal of Geriatrics Online Education. Is one statin superior to the others for use in the elderly? 25 March 2010. Available online at https://pogoe.org/ask/statins
15. New Zealand Formulary. Statins. Available online at https://nzf.org.nz/nzf_1599#nzf_1607
16. Davidson MH. Rosuvastatin in elderly patients. Drugs Aging 2007;24(11):933–44.
References (for abridged PDF)
1. Pharmac. Decision to fund rosuvastatin for people with high cholesterol. 17 August 2021. Available online at https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/2021-08-17-decision-rosuvastatin
2. Ministry of Health. Maori Health. Ngā mana hauora tūtohu: Health status indicators. Cardiovascular disease. Available online at www.health.govt.nz/our-work/populations/maori-health/tatau-kahukura-maori-health-statistics/nga-mana-hauora-tutohu-health-status-indicators/cardiovascular-disease
3. Ministry of Health. Pacific people's health. Available online at www.health.govt.nz/our-work/populations/pacific-health/pacific-peoples-health
4. Ministry of Health. 2018. Cardiovascular disease risk assessment and management for primary care. Wellington: Ministry of Health. Available online at www.health.govt.nz/system/files/documents/publications/cardiovascular-disease-risk-assessment-management-primary-care-feb18-v4_0.pdf
5. NPS Medicinewise. RADAR: Rosuvastatin (Crestor) for dyslipidaemia rosuvastatin. 1 December 2006. Available online at www.nps.org.au/radar/articles/rosuvastatin-crestor-for-dyslipidaemia
6. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 2003;92(2):152–60. Available online at www.sciencedirect.com/science/article/abs/pii/S0002914903005307
7. Tuomilehto J. Rosuvastatin: the most efficient treatment option for patients with dyslipidemia. Future Lipidology 2007;2(2):127–41. DOI: 10.2217/17460875.2.2.127. Available online at www.tandfonline.com/doi/pdf/10.2217/17460875.2.2.127
8. Jones PH, Hunninghake DB, Ferdinand KC, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther 2004;26(9):1388–99. Available online at www.clinicaltherapeutics.com/article/S0149-2918(04)80285-6/pdf
9. Medsafe. New Zealand data sheet. Rosuvastatin Viatris. Revised: 23 August 2021. Available online at www.medsafe.govt.nz/profs/Datasheet/r/rosuvastatinviatristab.pdf
10. NPS MedicineWise. APO-Rosuvastatin product information. Available online at www.nps.org.au/medicine-finder/apo-rosuvastatin#full-pi
11. Howe CL. POGOE: The Portal of Geriatrics Online Education. Is one statin superior to the others for use in the elderly? 25 March 2010. Available online at https://pogoe.org/ask/statins
12. Davidson MH. Rosuvastatin in elderly patients. Drugs Aging 2007;24(11):933–44.